
































To select the most suitable casting method, the following factors must be aligned with your requirements:
Surface quality
Dimensional accuracy
Number of cast parts
Type of pattern and core
Mold manufacturing cost
Limitations imposed by the selected material type

In the casting method, a solid meltable material is heated and then poured into a hollow cavity or mold to take the desired shape after solidification. As a result, in one step, it is possible to produce any complex or diverse shape from any meltable metal. The range of weight and size of parts that can be produced by casting is very wide, ranging from a one-millimeter part weighing less than one gram to large multi-ton parts with dimensions of several meters.
The casting process has significant advantages in manufacturing parts with hollow sections, complex shapes, internal cavities, very large parts, parts with irregular curved surfaces, and parts made of metals that are difficult to machine.

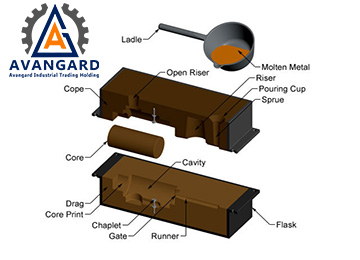
Sand Casting
In this method, grains of refractory material (such as silica) are mixed with small amounts of other materials like clay, binder, and water, and then compressed around a pattern that has the shape of the desired part.
Today, the design process in many industries, such as casting and modeling, automotive, aerospace, electronics, and more, is carried out through computers. This design method allows companies to first design their desired concept, then model and evaluate it. If the prototype model is approved, it is produced and put into operation.

Characteristics of Sand Casting
There are almost no limitations in shape, size, weight, or complexity, and any type of metal can be cast. Tolerances and surface finish are not as good as other casting methods, and some machining is usually required.
Main Characteristics of Sand Used for Sand Casting
1. Dry Strength
2. Green Strength: Shear and compressive strength in corners
3. Hot Strength: The mold quickly reaches high temperatures; it should not lose its shape when it loses moisture, as this can cause cracking, crumbling, or the formation of burrs and veins.
4. Gas Permeability: Gases released from the coating, binder, and air inside the mold must escape, which depends on the sand’s grain size, shape, moisture content, binder, and compaction.
5. Thermal Stability: Low thermal expansion coefficient and the ability to maintain dimensions.
6. Refractoriness: The mold material must be heat-resistant, not change state, burn, or melt.
7. Flowability: Depends on grain size and mesh.
8. Surface Quality (Produces Good Casting Finish): Depends on the physical properties of the sand grains.
9. Collapsibility: Depends on the type and amount of binder used.
10. Reusability
11. Easy preparation and control
12. Cooling Capacity (Remove Heat)

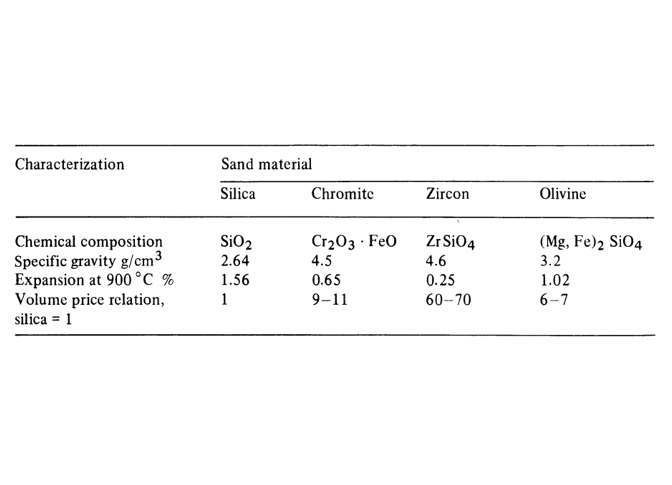
- Silica sand
- Chromite sand
- Olivine sand
- Zircon sand
- Chamotte sand

The chemical composition of silica sand is SiO2, and it is one of the most widely used and accessible types of sand in the casting industry. Typically, fine-grained sand is used for producing small-sized parts, while larger-grained sand is used for larger molds to ensure higher tensile strength, pressure resistance, and increased mold strength. Silica sand is commonly used in most sand castings because it is abundantly available in nature and is inexpensive and easy to access. To remove moisture in sensitive molds, some casters place sand molds in a mold-drying oven hours before pouring to completely dry the mold. Dry sand molds are stronger than green sand molds and offer higher dimensional accuracy. It is worth noting that the refractory limit of silica sand is up to 1730 degrees Celsius.
| Type of Sand | Chemical Composition | ||||||
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O | Na2O | |
| High-quality Silica Sand | 98 | 1.1 | 0.05 | 0.11 | 0.02 | 0.65 | 0.07 |
| Regular Silica Sand | 85 | 10 | 2 | 1 | 0.5 | 0.75 | 0.5 |
| Low-quality Red Silica Sand | 78 | 10 | – | 2.4 | 1.8 | 3.1 | 0.2 |

- Grade A consists of high-chromium chromite with a chromium-to-iron ratio of 2.5 and chromium content above 64%.
- Grade B consists of chromite with a chromium-to-iron ratio of 2.5–1.8



Chamotte sand with the chemical composition (3Al2O3.SiO2) is suitable for thick and large parts and is obtained by sintering clay. The advantage of this sand over silica is its uniformity and low volumetric changes due to temperature variations. Since it reacts chemically more slowly and adheres minimally to the surface of the part, it is used in steel casting. This sand has a refractoriness of 1670-1750°C, and the higher the Al2O3 content, the better. Chamotte sand is used in foundry applications for bricks and furnace linings.

Among the sands we have discussed, silica sand with high purity is the most widely used for sand casting operations. Some countries, such as Germany, Belgium, the Netherlands, and France, have sand of very high quality SiO2=99.8%, while other countries like Italy or Japan must import sand for high-quality iron and steel casting.
It is evident that when using sands with higher amounts of alkali oxides (K2O, CaO, Na2O, and MgO), a greater quantity of acidic curing agents is required.
The advantages of silica sands used in this industry include their low cost, availability in various grades, compatibility with different chemical binders, sufficient thermal and chemical resistance in contact with molten metals, and the ability to create molds and cores of various dimensions.
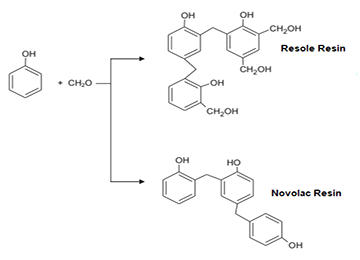
All phenolic, phenolic-urethane, furanic, and sodium silicate resins are used in the foundry industry as binders. Suitable resins used as binders and bonding agents for sands in this industry must possess the following characteristics:
- Short curing time
- Appropriate cost
- High dimensional stability
- Proper hardness and mechanical properties of the core
- High casting surface quality
- Desirable mold or core collapse
Phenolic resin, depending on the molar ratio of formaldehyde to phenol and the type of reacting catalyst used (which may be basic or acidic), is prepared in two forms: resol and novolac. Resol resin cures only with temperature, even without a curing agent (although the presence of a catalyst accelerates the curing time). However, novolac is a thermoplastic resin and requires a hexamine curing agent for cross-linking.
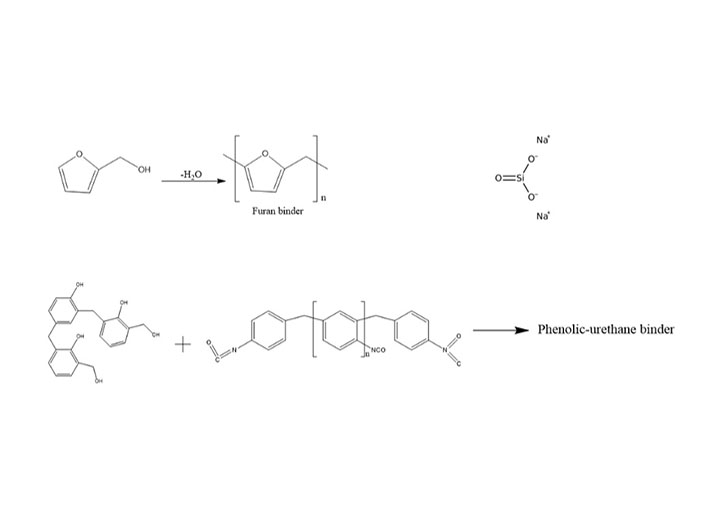
- Organic binders
- Inorganic binders
- Water-soluble or water-based
- Non-water-soluble or non-water-based
An optimal and good binder connects the sand grains together and increases the final strength of the mold and core in both dry and wet states. It must fulfill the following conditions:
- Spread uniformly on the surfaces of the base sand during the preparation of core or molding mixtures.
- Provide adequate strength to the sand mixture in both dry and wet states.
- Provide optimal and sufficient moldability to the mixture so that it can fill all parts of the mold.
- Have minimal adhesion to the model surface and core box.
- Allow for quick drying of the mold and core, and prevent moisture absorption by the mixture.
- Generate the minimum possible gas during pouring.
- Not reduce the refractoriness of the sand.
- Enable easy disintegration of the core after pouring.
- Not be harmful to individuals preparing the mold and core mixtures, and not produce toxic gases.
- Be inexpensive and readily available.
Core binders are very extensive and diverse, with many types available. Each of these binders is added to the core sand for a specific purpose and to provide particular properties. Most core binders (which are also used in mold making in many cases) cannot be reused after the first application due to structural changes. The binders used in core making can be divided into four groups:
1- Binders that harden upon freezing:Water is the only adhesive in this category used in foundry operations. In parts manufactured in Russia using this type of binder, it is claimed that internal cavities in the shells of the parts are eliminated using frozen cores.
2- Binders that harden at room temperature:Examples include sodium silicate, double silicates of calcium and aluminum, and cement. Detailed explanations about sodium silicate binder will be provided separately later. Among cement binders, rubber cement, Portland cement, and chemical cement can be mentioned, with Portland cement being the most important and widely used. A disadvantage of cores made with these cement binders is their low collapsibility.
3- Binders that harden after curing:These binders are further divided into three subcategories:
- 3-1- Binders that dry with heat (core oils):
In oils, hardening occurs through polarization and the formation of larger molecules by absorbing oxygen from the air at temperatures between 200 and 240°C. These binders consist of three types of oils: marine animal oils, vegetable oils, and mineral oils.
- 3-2- Binders that harden upon cooling after heating:
Various resins, or gums, fall into this category. Gums are classified into synthetic and natural types. Natural gums include tree sap. Urea-formaldehyde resins are the most widely used and offer the best characteristics for producing thin and small cores that require high collapsibility after pouring. Phenol-formaldehyde resins have lower collapsibility and are used for large steel castings. These resins are available in liquid and solid forms and should not be stored for extended periods.
- 3-3- Binders that gain adhesive properties under heat:
This group includes starch-based binders (dextrin), sulfide binders, protein binders, and beet molasses. Dextrins or starch-based binders are part of core binders and are typically used to increase the green strength of the core sand mixture. Adding 0.5 to 2% by weight increases the compressive strength of the sand by 1 to 2.5 pounds per square inch. Beet molasses is a byproduct of sugar factories and is usually added to cores along with other binders (except oil-based binders). Protein binders include commercial pure types such as casein, gelatin, and glue.
This group of binders is primarily added to the core sand mixture to increase its green strength. The most important ones are refractory clay (kaolin) and bentonite, which were also mentioned in molding binders.

In addition to the three main components of molding sand (sand, water, and binder), other materials are added to the mixture to improve quality and address specific defects. These materials are numerous, so here we mention some of the most commonly used ones:
1- Coal Dust: Fe2O3 produced in the molten iron products (cast iron, steel, etc.) reacts with the mold walls, which are often made of silica (a type of sand), forming fayalite. This reaction causes sand burning and sand sticking on the surface of the casting. Adding coal dust creates a reducing environment due to the combustion of coal and the release of CO2, hydrogen, and light hydrocarbons. This prevents oxidation of the molten surface and the formation of low-melting-point fayalite, ultimately preventing sand sticking and improving the smoothness of the casting surface.
Additionally, adding coal dust can prevent defects such as thermal expansion. The contraction of coal dust due to heat compensates for the expansion of sand caused by temperature increases. It also improves the sand’s crushability, as the sand clumps less and breaks down faster on shakeout.
Characteristics of an optimal coal dust are as follows: a) High volatile content (approximately 26-30%). b) The ash content should not exceed 10%. c) Another important parameter is sulfur content, as sulfur tends to cause chilling in gray cast iron. The sulfur content should not exceed 1%.
The amount of coal dust used for parts weighing up to 1000 kg is 2-4%, while for heavier parts with higher heat from pouring and solidification, and greater sand burning depth, the coal dust usage can reach up to 8%.
2- Cereals: The cereal binder used in foundry is gelatinized starch or fine powder derived from grains. Starch can be added to molding sand up to 2% to increase green or dry strength or improve collapsibility. Since starch is volatile, improper use can lead to gas defects in castings.
3- Wood Flour: Wood flour or other cellulosic materials like corn or grain flour, husks, and cellulose can be added to molding sand in amounts of 0.5-2%. This controls sand expansion. Cellulosic materials ignite upon heating and leave voids after burning, controlling the expansion of the molding sand mixture. Cellulosic materials and wood flour also improve flowability, making it easier to remove the casting from the mold.
4- Silica Flour: Finely ground silica with a mesh size of less than 200 is called silica flour. It can be added to sand up to 35% to increase hot strength. Silica flour increases the bulk density of the sand, making the molding sand more resistant to molten metal penetration due to higher compaction.
5- Iron Oxide: Iron oxide is used in small amounts in some molding sands to increase hot strength.
6- Molasses, Dextrin: Unrefined beet molasses or sugarcane molasses containing 60-70% sugar can be used to increase the dry strength of sand and improve the hardness of mold edges. Dextrin or starch gum can be used for the same purposes.
7- Perlite: Perlite is an expanded mineral aluminum silicate. It is used in small amounts (0.5-1.5%) to improve the thermal stability of sand. It can also be used for insulating feeder sleeves.
8- Asphalt: A byproduct of crude oil decomposition. Asphalt, like bitumen, is used to increase hot strength and improve the surface finish of iron castings.
9- Coal Tar Pitch: A product of coking. During coking, pitch is separated from coal at 350°C. Pitch is used up to 3% to improve hot strength and the surface quality of iron castings.
10- Gilsonite: A solid asphalt material mined in some parts of the world. It is volatile and functions similarly to coal in optimizing the surface finish of castings.
11- Fuel Oil: Fuel oil is sometimes used in very small amounts (0.01-0.1%) in molding sand mixtures to improve moldability.
12- Red Iron Oxide: Red iron oxide with an annual production capacity of 1500 tons is used in industries such as paint, metal primers, glazes, tiles, and ceramics. It is competitive with imported samples and has the following specifications:
Iron oxide for foundry cores: Black iron oxide with specific grain sizing according to customer requirements, composed of FeO, Fe2O3, and Fe3O4, is produced for use in foundry cores to achieve desirable properties.
The use of black and red iron oxide, as recommended by experts in engineered sand additives (ESA), has been tested in practical workshop conditions, and its effects have been observed. Iron oxide added to various sand mixtures has been tested for its impact on sand properties and its ability to reduce veining or wrinkling on casting surfaces.
Red iron oxide is effective in preventing wrinkles or veining on casting surfaces. However, due to its powdery nature and lack of grain sizing, it can have adverse effects on the surface finish. Black iron oxide, added at 3% in hot box systems and 2% in cold box systems, is effective in eliminating veining or wrinkling and has less detrimental impact on surface quality. Other reasons for using iron oxide include reducing carbon sticking to the casting, preventing pinholing, and resisting iron penetration into the mold.
In general, the effects of iron oxide in core making and its purposes are as follows:
a) Purpose of use: Increases hot plasticity and reduces or eliminates veining, metal penetration, and gas defects.
b) Effects of iron oxide in core making: 1- Increases the dry strength of produced cores. 2- Reduces the effects of nitrogen and hydrogen pinholes. 3- Effectively controls thermal expansion of the core. 4- Enhances the hot strength of the core. 5- Prevents molten metal penetration into the core and forms a suitable, hard fayalite layer on the core surface, preventing nitrogen gas from the hot box resin from entering the molten metal. 6- Controls the mold atmosphere by releasing oxygen, protecting it from excessive reduction and improving surface quality.
13- Tin Powder and Tin-Antimony Powder: Used in mold coatings for cast iron in contact with resin cores due to their effect on the surface structure. Tin and tin-antimony intermetallic compounds stabilize the pearlite phase and eliminate ferritic surface layers.
14- Dextrin: Dextrin is a type of hydrocarbon gum derived from the decomposition of starch by acids, heat, or enzymes. It is used to increase the strength of molding sand or cores and in mold coatings.

One of the most widely used methods for preparing molding sand in foundry is the use of sodium silicate binder and carbon dioxide gas (CO2) in the sand mixture. In this method, sodium silicate binder and CO2 gas are used to harden the sand particles, which is why it is commonly known as CO2 sand in the foundry industry. This method is widely used in the Iranian foundry industry for mold and core making. The process works as follows: during the preparation of the sand mixture for molding, a specific amount of sodium silicate binder is added to the sand and thoroughly mixed using a mixer. The sand-binder mixture is then transferred into the mold, and the sand ramming process is carried out.

The use of sodium silicate binder in the foundry industry dates back to around 1930. In 1960, the use of CO2 gas became common. The invention of this method revolutionized the foundry industry. This method can be used for casting iron, steel, and non-ferrous metal parts.
Sodium silicate binder is added to the silica sand mixture at 3 to 5% by weight. The amount of sodium silicate binder added depends on factors such as sand grain size, type, shape, alloy type, required strength, pouring temperature, mold or core collapsibility, and the mold’s resistance to wear and breakage. The finer the sand grain size, the higher the binder consumption. After adding the binder to the sand, they are mixed until the binder is uniformly distributed in the sand and no clumps remain. Excessive use of the binder leads to higher CO2 consumption, reduced mold strength over time, and the mixture becoming porous and powdery.
Analysis of Sodium Silicate Binder
| Brand | Ratio | Na2O% | SiO2% | Solid Content, % | Density, 25°C (ba) | Specific | Viscosity (Cps) | Grade | Appearance | Addition |
| A.I.T.H 25 | 2.5 | 13.52 | 32.85 | 46.37 | 52 | 1.551 | 886 | Ceramic | Hazy | 2% Molasses |
Mixing and preparing the sand and sodium silicate binder is done in continuous or batch mixers. In batch mixers, a specific amount of binder and sand is poured into the mixer, mixed, and the sand is ready for use. In continuous mixers, the machine operates continuously. Binder and sand are fed into the chamber from separate hoppers, mixed, and then discharged from the other side.

After preparing the sand, the mold or core is made using this sand-binder mixture. The sand must be used as quickly as possible. Typically, CO2 gas is injected into the sand at a pressure of 1-3 bar for 5 to 60 seconds. To ensure the gas penetrates all parts of the mold, the mold is punctured with a rod, and the gas nozzle is inserted into the hole to complete the gassing process. When CO2 gas is blown into the mold, an immediate reaction occurs between the carbonic gas and sodium silicate. This reaction causes silica to form a silica gel (gelatinous silica), bonding the sand grains together.

The initial strength of cores and molds gassed for 5 seconds is 255-310 kPa. However, the strength increases over time, reaching 670-1380 kPa after 24 hours. The reason for this strength increase over time is the dehydration of unreacted silicate and the continuation of the silica gelation process. CO2 gas can harden an area with a radius of 70 mm around the gassing hole. Therefore, it is recommended that the distance between two gassing holes in the sand mold should not exceed 140 mm.

First, half of the pattern is placed in the lower flask. Then, sand is poured over the pattern and compacted using specialized tools to take the shape of the pattern.

After gassing to strengthen the mold, the lower flask is flipped, and the upper flask is placed on top. The other half of the pattern is assembled on it, and after placing the feeders and gating system, the upper flask is also filled with sand, compacted, and gassed again. Finally, the two flasks are separated, and the wooden or metal patterns, along with the gating and feeder patterns, are removed from the sand. The paths of the gating system are smoothed using specialized tools, and their surfaces are finished.

Then, the mold surface is coated with a suitable thickness of refractory coatings for the following reasons:
When molten metal at high temperatures is poured into the sand mold, destructive reactions occur between the molten metal and the sand or binder, leading to defects such as sand burning, roughness, and unevenness on the surface of the casting. In many cases, these defects render the part unusable. Issues like sand burning, erosion, washing away of sand, and chemical interactions between the molten metal and the sand fall into this category. However, if a suitable refractory coating is applied to the sand surface in contact with the molten metal, such defects can be significantly reduced or eliminated.
Refractory coatings are a special type of paint used in sand casting. They differ from ordinary paints in that they are designed to withstand the high temperatures of molten metal and act as a barrier between the molten metal and the sand mold or core surface. Common methods of applying the coating include spraying with a spray gun or brushing.

After the mold and coating have completely dried, the core is placed inside the mold.

Finally, the mold is assembled and ready for pouring.


 فارسی
فارسی